
Ammonium sulfate
Name:Ammonium sulfate
Molecular Structure
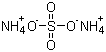 Molecular Formula :(NH4)2.SO4;H8N2O4S
Molecular Weight :132.13
CAS Registry Number :7783-20-2
EINECS :231-984-1
Ammonium sulfate, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen as ammonium cations, and 24% sulfur as sulfate anions. In fertilizer the purpose of the sulfate is to reduce the soil pH.
Ammonium sulfate is used largely as an artificial fertilizer for alkaline soils. In the soil the sulfate ion is released and forms bisulfate, lowering the pH balance of the soil (as do other sulfate compounds such as aluminium sulfate), while contributing essential nitrogen for plant growth.
Ammonium sulfate is also used as an agricultural spray adjuvant for water soluble insecticides, herbicides, and fungicides. There it functions to bind iron and calcium cations that are present in both well water and plant cells. It is particularly effective as an adjuvant for 2,4-D (amine), glyphosate, and glufosinate herbicides.
It is also used in the preparation of other ammonium salts.
In biochemistry, ammonium sulfate precipitation is a common method for purifying proteins by precipitation. As such, ammonium sulfate is also listed as an ingredient for many United States vaccines per the Center for Disease Control.
Ammonium sulfate is also a food additive.
A saturated solution of ammonium sulfate in heavy water (D2O) is used as an external standard in sulfur (33S) NMR spectroscopy with shift value of 0 ppm.
It has also been used in flame retardant compositions acting much like Diammonium phosphate. As a flame retardant, it lowers the combustion temperature of the material, decreases maximum weight loss rates, and causes an increase in the production of residue or char.
Specification:
Packing: In 25/50kg net PP/PE bag or the demanding of customer. |