
Fentanyl Citrate
Name: Fentanyl citrate
Synonyms: N-(Phenyl)-N-(1-[2-phenylethyl]-4-piperidinyl)propanamide citrate
Molecular Structure:
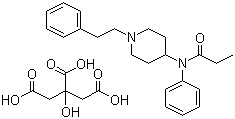 Molecular Formula: C22H28N2O.C6H8O7
Molecular Weight: 528.59
CAS Number: 990-73-8
EINECS: 213-588-0
Fentanyl (fentanyl citrate) is a synthetic primary ¦Ì-opioid agonist commonly used to treat post-operative and chronic breakthrough pain. Fentanyl citrate is approximately 100 times more potent than morphine, with 100 micrograms of Fentanyl equivalent to 10 mg of morphine and 75 mg of pethidine (meperidine) in analgesic activity. Fentanyl citrate has an LD50 of 3.1 milligrams per kilogram in rats, 0.03 milligrams per kilogram in monkeys, and an undetermined milligrams per kilogram in humans. Fentanyl citrate was first synthesized by Dr. Paul Janssen in 1959 following the medical inception of meperidine several years earlier, and based initially on the chemical starting point of piperidine and applicable opioid activity. The widespread use of Fentanyl citrate triggered the production of fentanyl citrate, which entered the clinical practice as a general anaesthetic under the trade name Sublimaze in the 1960's. Following this, many other fentanyl analogs were developed and introduced into the medical practice, including Sufentanil, Alfentanil, Remifentanil, Carfentanil, and Lofentanil. In the mid 1990s, Fentanyl citrate saw its first widespread palliative use with the clinical introduction of the Duragesic patch, followed in the next decade by the introduction of the first quick-acting prescription formations of fentanyl for personal use. Through the delivery method of transdermal patches, Fentanyl citrate is currently the most widely used synthetic opioid in clinical practice, with several new delivery methods currently in development. Fentanyl citrate is classified as a Schedule II drug in the United States due to its high potential for abuse.
Fentanyl citrate was first synthesized by Paul Janssen under the label of his relatively newly formed Janssen Pharmaceutica in 1959. In the 1960s, fentanyl was introduced as an intravenous anesthetic under the trade name of Sublimaze. In the mid-1990s, Janssen Pharmaceutica developed and introduced into clinical trials the Duragesic patch, which is a formation of an inert alcohol gel infused with select fentanyl doses which are worn to provide constant administration of the opioid over a period of 48 to 72 hours. After a set of successful clinical trials, Duragesic fentanyl patches were introduced into the medical practice.
Following the patch, a flavored lollipop of fentanyl citrate mixed with inert fillers was introduced under the brand name of Actiq, becoming the first quick-acting formation of fentanyl for use with chronic breakthrough pain. More recently, Fentanyl citrate has been developed into an effervescent tab for buccal absorption followed by a buccal spray device for fast-acting relief and other delivery methods currently in development. |