
Diethylstilbestrol
Name: Diethylstilbestrol
Synonyms: (E)-3,4-Bis(4-hydroxyphenyl)-3-hexene; (E)-4,4'-(1,2-Diethyl-1,2-ethenediyl)bisphenol; DES
Molecular Structure:
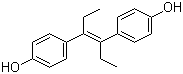 Molecular Formula: C18H20O2
Molecular Weight: 268.35
CAS Number: 56-53-1
EINECS: 200-278-5
Diethylstilbestrol (DES) is a drug, an orally active synthetic nonsteroidal estrogen that was first synthesized in 1938. In 1971 Diethylstilbestrol was found to be a teratogen when given to pregnant women. DES (in tablets up to 5 mg) was approved by the United States Food and Drug Administration on September 19, 1941 for four indications: gonorrheal vaginitis, atrophic vaginitis, menopausal symptoms, and postpartum lactation suppression to prevent breast engorgement. The gonorrheal vaginitis indication was dropped when the antibiotic penicillin became available.
DES has been very successful in treating female canine incontinence stemming from poor sphincter control. Diethylstilbestrol is still available from compounding pharmacies, and at the low (1 mg) dose, does not have the carcinogenic properties that were so problematic in humans. Diethylstilbestrol is generally administered once a day for five days and then once every 4 to 7 days as needed. During the 1960s DES was used as a growth hormone in the beef and poultry industry. |