
Fumaric acid
Name :Fumaric acid
Synonyms :(E)-2-Butenedioic acid; Butenedioic acid; (E)-1,2-Ethenedicarboxylic acid; TMEDA
Molecular Structure
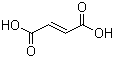 Molecular Formula: C4H4O4
Molecular Weight: 116.07
CAS Registry Number: 110-17-8
EINECS: 203-743-0
FEMA :2488
Fumaric acid, when added to food products, is an acidity regulator denoted by E number E297. Fumaric acid is found in fumitory (Fumaria officinalis), bolete mushrooms (specifically Boletus fomentarius var. pseudo-igniarius), lichen, and Iceland moss.
Fumarate is an intermediate in the citric acid cycle used by cells to produce energy in the form of adenosine triphosphate (ATP) from food. It is formed by the oxidation of succinate by the enzyme succinate dehydrogenase. Fumarate is then converted by the enzyme fumarase to malate. Human skin naturally produces fumaric acid when exposed to sunlight. Fumarate is also a byproduct of the urea cycle.
Fumaric acid esters are sometimes used to treat psoriasis, as it has been suggested that the condition is caused by an impairment of fumaric acid production in the skin. A starting dose is 60-105 mg daily, which may be gradually increased to as much as 1,290 mg per day. Side-effects include kidney or gastrointestinal disorders, as well as skin flushing; these are mainly caused by excess intake. Decreased white blood cell (WBC) counts have been reported with prolonged use.
Fumaric acid is a food acidulent used since 1946. It is non-toxic. It is generally used in beverages and baking powders for which requirements are placed on purity. It is generally used as a substitute for tartaric acid and occasionally in place of citric acid, at a rate of 1.36 g of citric acid to every 0.91 grams of fumaric acid for the same taste. It is also used in candy to add sourness, similar to the way malic acid is used.
Fumaric acid was first prepared from Succinic acid. A traditional synthesis involves oxidation of furfural (from the processing of maize) using sodium chlorate in the presence of a vanadium-based catalyst. Nowadays industrial synthesis of fumaric acid is mostly based on catalytic isomerisation of maleic acid (which in turn is accessible in large volumes as a hydrolysis product of maleic anhydride, produced by catalytic oxidation of benzene or butane) in aqueous solutions.
The chemical properties of fumaric acid can be anticipated from its component functional groups. This weak acid forms a diester, it undergoes additions across the double bond, and it is an excellent dienophile.
Specification: (FCCIV, 1996)
Package: 25kg net in plastic woven bag lined with PE bag. 18 MT/20'FCL (with pallet) or 20MT/20'FCL (without pallet). 500kg-1000kg container bags of all specifications are available.
Storage: Store in cool and dry place.
Shelf life:2years
|