
Ciprofloxacin
Name: Ciprofloxacin
Synonyms: 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid
Molecular Structure:
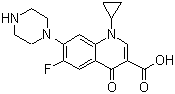 Molecular Formula: C17H18FN3O3
Molecular Weight: 331.34
CAS Number: 85721-33-1
Ciprofloxacin (INN) is a chemotherapeutic agent used to treat specific bacterial infections. Ciprofloxacin is a second generation fluoroquinolone antibacterial. Ciprofloxacin is marketed worldwide; with well over three hundred different brand names. In the United States, Canada and the UK, Ciprofloxacin is marketed as Ciloxan, Cipro, Cipro XR, Cipro XL Ciproxin and, most recently, Proquin. In Mexico Ciprofloxacin is available over the counter and marketed under the names Ciproflox or Ciprofloxacino. In Ecuador Ciprofloxacin is available and marketed under the name Cidrax. Additionally, ciprofloxacin is available as a generic drug under a variety of different brand names and is also available for limited use in veterinary medicine. Ciprofloxacin was first patented in 1983 by Bayer A.G. and subsequently approved by the United States Food and Drug Administration (FDA) in 1987.
The licensed uses for ciprofloxacin in the United States are quite limited as ciprofloxacin is to be considered a drug of last resort when all other antibiotics have failed. These consist of ten approved uses in the adult population and two approved uses in the pediatric population, as well as a variety of veterinary uses (as documented within the package inserts). As with any drug, Ciprofloxacin is oftentimes prescribed for unapproved uses (off label i.e. uses that have not been approved by the regulator authorities) Ciprofloxacin interacts with a number of other drugs, a number of herbal and natural supplements, and certain thyroid medications. |